Applications (a partial list):
- In vivo Optical Imaging of Intrinsic Signals (OIIS) in the Cortex
- Widefield fluorescence imaging of Genetically Coded Indicators
- In vivo real-time optical imaging of cortical activity using Voltage Sensitive Dyes
- Optical Inspection
- Inspection of Optical Components under Polarized Light
Low Magnification Imaging can be challenging under low-light conditions:
Low-magnification systems are desirable when it is important to image relatively large Fields-of-View, typically of a size that is of the order of the size of commercially available image sensors (in the range of 11mm ~ 22mm).
While it is possible to use Low-magnification microscope objectives to image large Fields-of-View, such objectives typically have small Numerical Apertures. A typical microscope objective with a magnification of 1.0x may have an N.A. of 0.05; this limits the ability of low-magnification systems to capture light under low-light conditions. The light collection of an optical system is typically proportional to the square of its N.A. This means that all else being equal, an optical system with an N.A. = 0.05 could theoretically collect only 1% of the light that would be collected by a system with N.A. = 0.5 (a rather serious problem under light starved conditions).
A simple solution: the Tandem-Lens Macroscope configuration:
A Tandem-Lens Macroscope essentially comprises of two camera lenses with the front optical surfaces of the two lenses facing each other and held via an adapter that uses the filter-threads of each lens. One of the lenses is inserted into a camera in the normal fashion (using adapters if needed). This lens is referred to as the imaging lens. The other lens faces the reverse direction and is referred to as the reversed lens or the objective lens.

Features of a front-coupled Tandem-Lens Macroscope configuration:
- A tandem-lens macroscope with lenses of smaller f/#s provide higher NA
- Excellent light collection
- High N.A. systems have a shallow Depth of field (DoF), which may be considered to give them an optical “sectioning” capability
- Working distance is determined by the flange focal distance of the lens that is used as the “objective”
Notes:

- The lenses may or may not be identical
- The focus ring of both lenses should be set to ꝏ and the lens iris ring (f/#) would typically be set to the lowest number (iris = wide open)
- Magnification is determined by the ratio of the focal lengths of the two lenses that are used.
- Magnification = M = FLimaging/FLobjective
- Depth of Field = DoF ≈ 2 x CoC/M2 x (fimaging + Mfobjective)
- CoC = circle of confusion specified by the experimenter: the minimum acceptable defocus in the imaging plane
- In the DoF equation fimaging and fobjective are the f/#s of the two lenses, not their focal lengths
- If two identical 50mm, f/1.2 lenses are front-coupled and 10µm is chosen by an experimenter as the CoC:
- Magnification: M = FLimaging/Flobjective= 50mm/50mm = 1.0x
- DoF ≈ 2 x 10µm/1.02 x (1.2 + 1.0 x 1.2) = 48µm
A simple implementation (as shown in Figure 2):
- Objective Lens: an f/1.4 lens with a focal length = 85mm
- Imaging Lens: an f/2.0 lens with a focal length = 105mm
- Long Working Distance: > 40mm
- Low magnification: M = FLimaging/Flobjective= 105mm/85mm = 1.24x
- Zoomable lenses allow FOVs of 17.1mm x 14.4mm ~ 12.5mm x 10.5mm; ideal for imaging the whole cortex of adult mice
- High NA: ~ 0.36, vs. NA of ~0.05 with conventional low-magnification microscope objectives
- If a CoC of 10µm is chosen => DoF ≈ 2 x 10µm/1.242 x (2 + 1.24 x 1.4) = 48.6µm
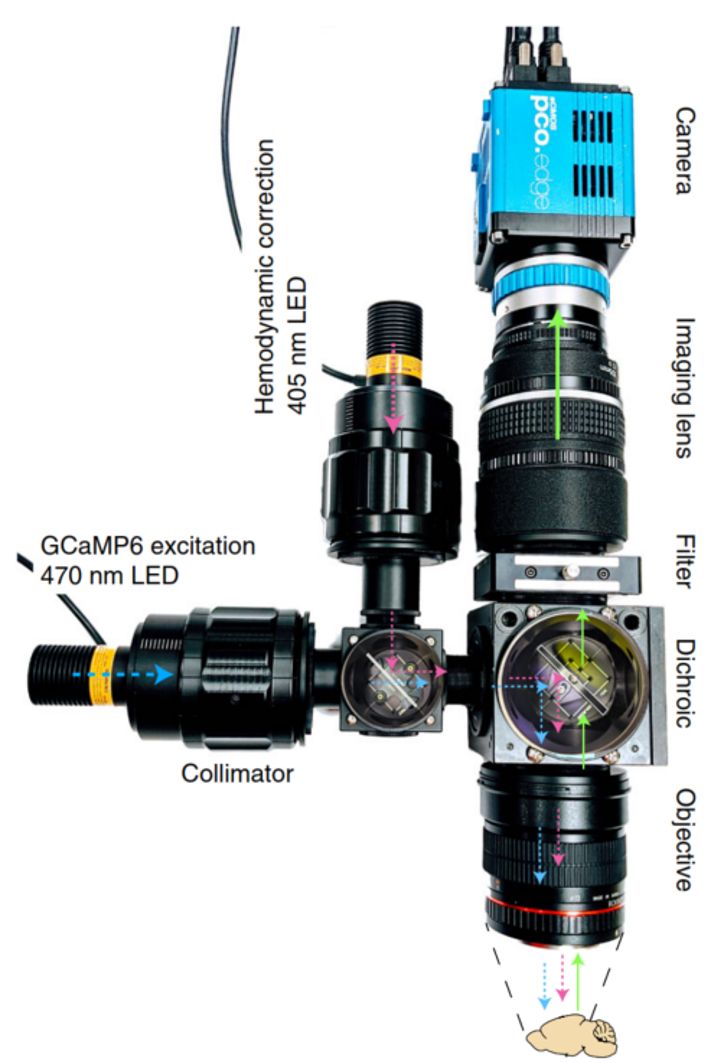
In Vivo widefield fluorescence imaging of cortex (as shown in Figure 3):
- Ref: Couto, J., Musall, S., Sun, X.R. et al. Chronic, cortex-wide imaging of specific cell populations during behavior. Nat Protoc 16, 3241–3263 (2021)
- The above Nature Protocols paper describes the use of a tandem-lens macroscope in an often cited Churchland Laboratory protocol optimized for imaging green fluorophores, e.g GCaMP6
- Modified tandem-lens macroscope with a filter-cube introduced in the so-called “infinity space” between the lenses
- Note that in an epi-fluorescence application the level of excitation as well as the light collection of the imaging path are each proportional to the square of the N.A. This means that such applications derive even more benefit from the high N.A. of a tandem-lens configuration.
- The sketch shows two triggerable/switchable illumination paths
- Blue (470nm): excites GCaMP6 + nonneuronal hemodynamic signals
- Violet (405nm): excites nonneuronal signals but not GGaMP6
- Scaling and processing allows extraction of neuronal signals from sequentially captured images resulting from alternating Blue/Violet excitation
- Scientists may select from a range of sCMOS cameras that are ideal for this type of imaging due to their high dynamic range, low noise and large Field-of-View
- As described in the Nature Protocols paper, the authors selected a PCO edge 5.5 sCMOS camera due to its high dynamic range, low noise and large Field-of-View.
- The paper provides links to downloadable code that was developed to support this camera.
- Researchers who are seeking to emulate this protocol are invited to contact Scientific Imaging, Inc. for details.
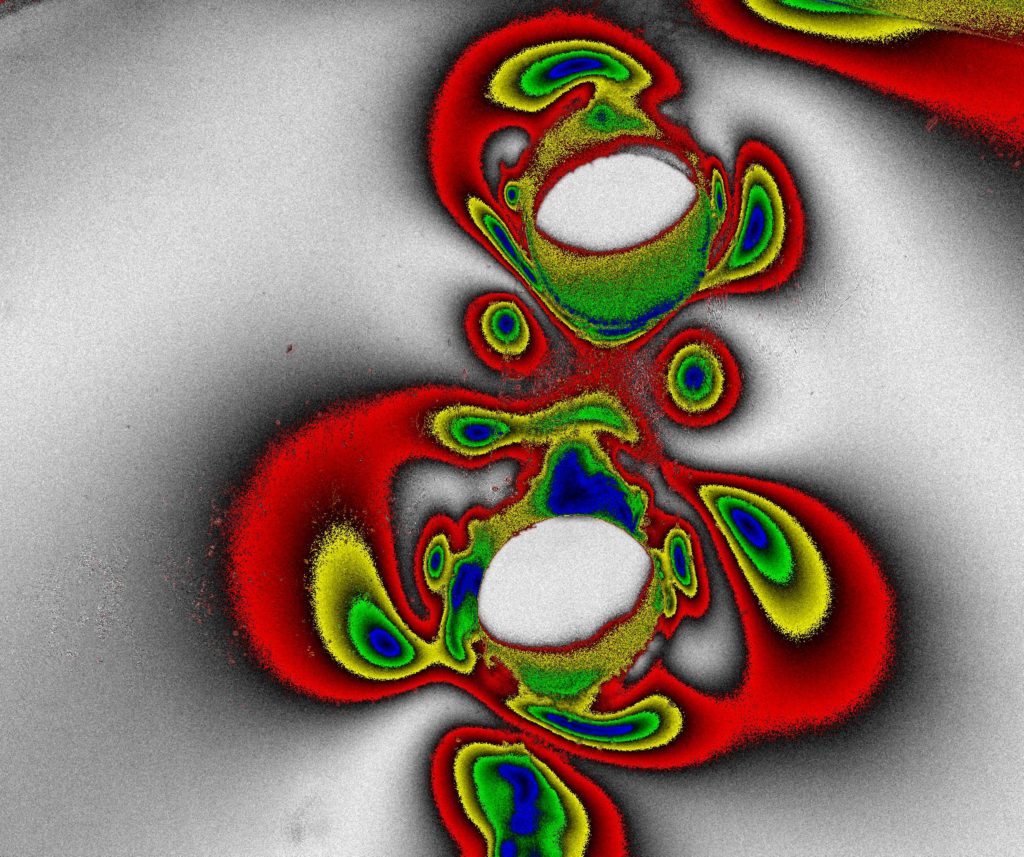
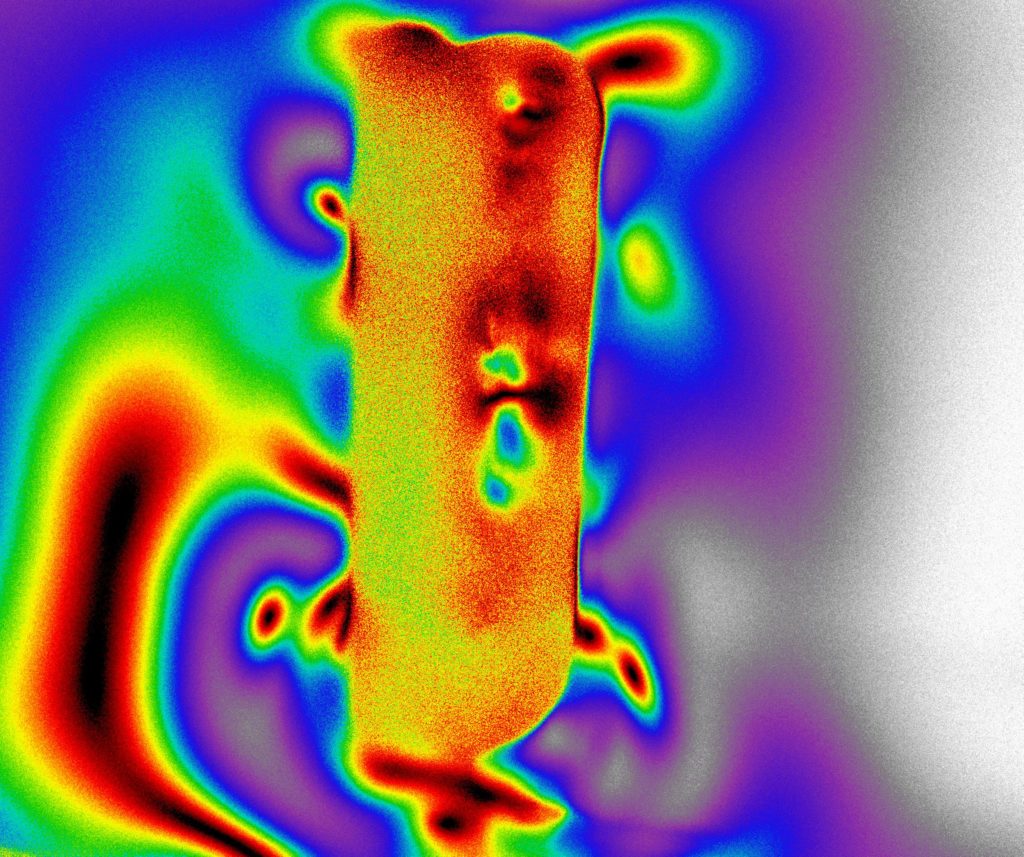
Inspection of Transparent Materials using the Tandem-lens macroscope configuration:
The shallow DoF and excellent light collection of a Tandem-lens macroscope makes it a good choice for inspecting transparent materials such as plastic optics, film, glass, Liquid Crystal Displays, etc. Users can achieve a type of “optical sectioning” by z-stepping through layers of transparent material, acquiring in-focus images at each z-position within the limits of the DoF (typically < 50µm).
This technique is conducive to imaging a wide range of materials, using different wavelengths of interest and illumination techniques that are optimized specifically to the materials under inspection.
The following is a partial list of techniques:
- Brightfield illumination
- Darkfield/oblique illumination
- Fluorescence, using either reflected or transmitted illumination
- Polarized Light techniques, for which a polarizer-array-equipped 5MPix CMOS camera is highly recommended.
References:
- A tandem-lens epifluorescence macroscope: hundred-fold brightness advantage for wide-field imaging: Eugene H. Ratzlaff, Amiram Grinvald (1996).
- Functional Optical Imaging of Intrinsic Signals in Cerebral Cortex: L. E. Hallum and S. Chen and S. L. Cloherty and J. Morley and G. Suaning and N. Lovell (2006).
- Chronic, cortex-wide imaging of specific cell populations during behavior; Couto, J., Musall, S., Sun, X.R. et al. Nat Protoc 16, 3241–3263 (2021).
Please contact us to discuss your “tandem-lens macroscopy” needs.

