Dr. Ernst Stelzer (Goethe Universitat, Frankfurt am Main) outlines in this iBiology video, the advantages of a technique called Light Sheet Fluorescence Microscopy (LSFM).
LSFM is a fluorescence microscopy technique that is used with transparent (often clarified) samples, and permits the optical sectioning of such samples at high speed. Unlike traditional epifluorescence microscopy, a thin slice (usually a few hundred nanometers to a few micrometers) of the sample is illuminated in a plane that is perpendicular to the imaging path. A laser light-sheet is created by focusing a laser beam in one orientation (e.g. by using a cylindrical lens).
By limiting the illumination to one thin section at a time, the photodamage and stress induced on living cells is greatly reduced. The optical sectioning capability also reduces the background signal and thus creates images with higher contrast, comparable to confocal microscopy. LSFM involves the illumination (via laser light sheet) and imaging (via 2D image sensors in a camera) of a selected plane of instead of a point (as in confocal microscopy). For this reason it can acquire images at speeds 100 to 1000 times faster than those offered by point-scanning methods.
In this iBiology video, Dr. Hari Shroff (NIH) describes a special version of LSFM (see above video). Dual-View Inverted Selective Plane Illumination or diSPIM overcomes some of the disadvantages of LSFM, which Dr. Shroff describes in the first part of his lecture.
The disadvantages are overcome by using two objectives that are orthogonal and which can be mounted to a conventional microscope. By using one as an “illumination” objective and the other as an “imaging” objective and mounting both such that they are 45 degrees with respect to the sample, which is on a cover slip.
Dr. Shroff describes this as the evolution of iSPIM, which was an improvement on the previous LSFM technique, but suffered from the fact that the axial resolution of a microscope is about 3x worse than its lateral resolution. Note: this is detailed in the lectures on this page.
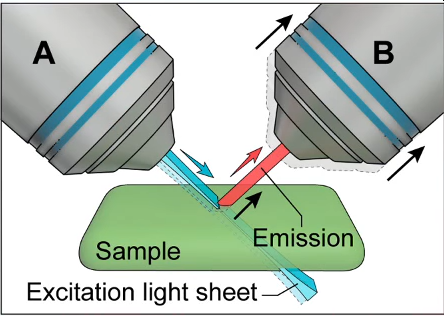
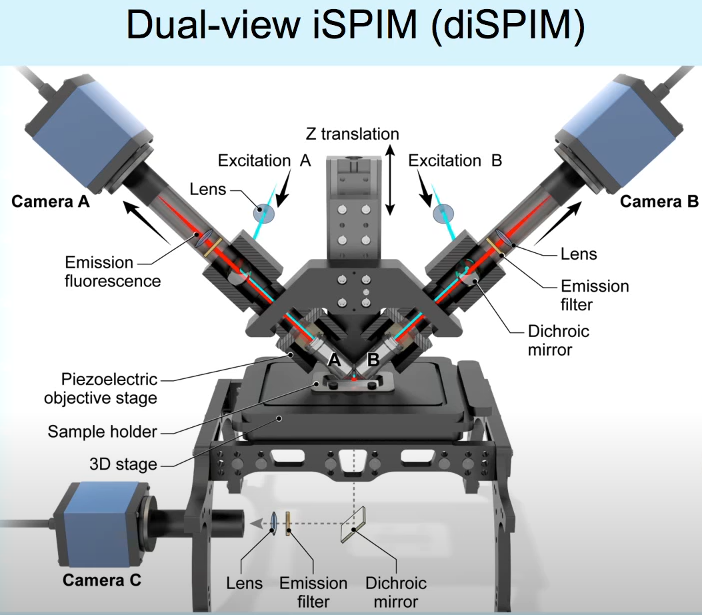
To develop a microscope that is capable of isotropic resolution, i.e. a system that has the same resolution in both lateral and axial views, the concept of diSPIM was developed. In a diSPIM system the illumination and imaging objectives simply swap roles alternately. Volumes of information from both orientations are collected and then computationally fused together. This doesn’t involve a significant change to the iSPIM system, except for the addition of a 2nd camera.
In this iBiology video, Dr. Daniel Axelrod (Emeritus, University of Michigan) elucidates the concept of Total Internal Reflection Fluorescence (TIRF) Microscopy.
TIRF is used to selectively illuminate fluorophores that are at or near the surface of a solution and not those that are away from the surface. This is useful in single molecule studies, where the intent is to see the molecules at the surface, while suppressing background fluorescence. It is also used in live cell imaging in culture, to view the cell/substrate contact region, but it is helpful to suppress the background fluorescence from further in the cell.
Dr. Philippe Bastiaens (Max Planck Institute of Molecular Physiology) discusses Forster Resonance Energy Transfer (FRET) microscopy in this iBiology video.
Dr. Bastiaens begins with the essential principles of FRET and then details the main parameters that apply to this imaging technique. He then elaborates on the main FRET techniques and the tradeoffs between their respective ease-of-use and their quantitative nature: Ratio imaging, Sensitized Emission, Acceptor Photobleaching and Fluorescence Lifetime Imaging
In this iBiology video Dr. Jennifer Lippincott-Schwartz (NIH) describes how fluorescence recovery after photobleaching (FRAP), fluorescence loss in photobleaching (FLIP) and photoactivation of fluorophores be used as tools for observing the dynamics of protein-dynamics within cells.
She provides details as to how FRAP and FLIP are useful techniques for imaging fluorescent proteins within cells. These techniques can give us new insights into how these molecules behave inside living cells.
Cameras selection for Advanced Fluorescence Microscopy techniques: sCMOS Cameras

